- New stuff to read and discuss
- What patients say
- Clinic / online appointments
- Why the diagnosis of a psychosomatic illness is often a misdiagnosis
- Vascular Compression Syndromes
- Do you have questions?
- Checklist vascular compression syndromes
- Description of your symptoms
- Researchers from the Mayo Clinic confirm my concept of the Midline Congestion Syndrome
- Explanation of gender-specific differences in the clinical symptoms of abdominal vascular compression syndromes: varicocele and penile/testicular pain – their main manifestation in men.
- Varicocoele is predominantly caused by left renal vein compression
- Musculoskeletal pecularities of female puberty
- Lordosis /Swayback- Origin of many abdominal compression syndromes
- Bending of a straight vein compels its narrowing
- The lordogenetic midline congestion syndrome
- Neurological consequences of the midline congestion syndrome
- Successful treatment of a teenage girl who was unable to eat due to extreme postprandial pain and unable to walk due to spasticity in her left leg
- Severe ataxia in a young woman with severe spinal congestion – complete resolution after decompression of the left renal vein
- All compression syndromes are one: the spectrum of lordogenetic compressions
- Nutcracker-Syndrome is a misnomer! Lordogenetic left renal vein compression is a more appropriate name!
- May-Thurner-constellation (May-Thurner-syndrome, Cockett’s syndrome)
- Midline (congestion) syndrome
- Pelvic congestion syndrome
- Celiac Trunk Compression / Dunbar syndrome / MALS / Arcuate ligament syndrome
- Wilkie-Syndrome / Superior-mesenteric-artery-syndrome
- Compression of the vena cava inferior
- Evlauation of vascular compressions with the PixelFlux-method
- Connective tissue disorders predispose to multiple compressions
- Postural tachycardia syndrome (POTS) – the hemodynamic consequence of vascular compression syndromes and loose connective tissue
- Restless legs-a little known symptom of abdominal vascular compression syndromes
- Pudendal neuralgia in vascular compression syndromes
- A new sonographic sign of severe orthostatic venous pooling
- Migraine and Multiple Sclerosis
- Hemodynamic effect on cerebral perfusion in patients with multiple localised vascular compression.
- Treatment of vascular compression syndromes
- Fatal errors in the treatment of vascular compression syndromes
- Risks of stents in venous compression syndromes
- Surgical treatment of abdominal compression syndromes: The significance of hypermobility‐related disorders
- Nutcracker and May-Thurner syndrome: Decompression by extra venous tube grafting and significance of hypermobility related disorders
- Our surgical treatment of vascular compressions
- Chronic regional pain syndrome (CRPS) caused by venous compression and mechanical irritation of the coeliac plexus
- Vascular compression syndromes I recently detected
- Kaleidoscope of instructive cases
- Ultrasound Diagnostics
- Profile
- Functional colour Doppler ultrasound – how I do it
- Perfusion Measurement – PixelFlux-method
- Research
- Publications
- Nutcracker and May-Thurner syndrome: Decompression by extra venous tube grafting and significance of hypermobility related disorders
- Papers authored by Th. Scholbach
- Publications
- Inauguration of measurements of the tissue pulsatility index in renal transplants
- From nutcracker phenomenon to midline congestion syndrome and its treatment with aspirin
- First sonographic tissue perfusion measurement in renal transplants
- First sonographic bowel wall perfusion measurement in Crohn disease
- First sonographic renal tissue perfuison measurement
- First sonographic measurement of renal perfusion loss in diabetes mellitus
- PixelFlux measurements of renal tissue perfusion
- Why I prefer not to publish in journals but in the Internet
- Vessel stretching in nephroptosis – an important driver of complaints
- Publications
- Expertise
- Bornavirus Infection
- Scientific cooperation
- Cookie Policy
- Data protection
- Cookie Policy (EU)
- Impressum

A rare variant of Wilkie syndrome
A rare variant of Wilkie syndrome
(published on 20.01.2024 on the 41st birthday of Prof. Dr. rer. nat. Jakob Scholbach, who developed the first version of the PixelFlux software as a pupil at the Ostwald-Gymnasium in Leipzig)
Wilkie syndrome (SMA syndrome, superior mesenteric artery syndrome) is a compression of the duodenum by the superior mesenteric artery when it crosses the aorta. This arterial clamp constricts the duodenum to such an extent that shortly after ingestion of food, the transportation of food immediately behind the stomach outlet (after 6 cm) stops and the food is pressed against this clamp, causing severe pain. The duodenum often expands to a diameter of 3 or more centimeters – which causes the pain – and the food spills back into the stomach at the end of a peristaltic contraction wave, often even into the mouth.
As a result, patients vomit, regurgitate, the stomach acid flows back, they have severe wave-like pain in the right upper abdomen and on the line connecting the navel and sternum when the futile contraction waves are thrown back at the arterial clamp. Finally, patients may suffer a potentially life-threatening loss of weight.
Unfortunately, this clinical picture, especially in young women and girls, is diagnosed as anorexia nervosa (psychogenic eating disorder) without a thorough physical examination. The consequences for the patients are devastating. They are accused of having severe psychological conflicts as the cause of their complaints, the pain is not believed, the complaints are only imagined and the vomiting is induced arbitrarily in order to lose weight.
In my opinion, the diagnosis of anorexia without ruling out vascular compression is a major mistake.
I would now like to present a previously undescribed variant of this clinical picture.
The very slim young patient (BMI minimum 15.5 – currently 18.7 under tube feeding) had a hypermobile Ehlers-Danlos syndrome.
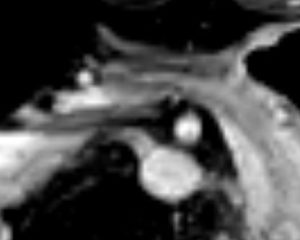
 After feeding, the filling stomach pushed the superior mesenteric artery further to the right to a distance of 23 mm from the aortic axis. Now the filling stomach pushed the superior mesenteric artery to a minimal distance of 7.12 mm to the anterior edge of the lumbar vertebral body. A space of about 3 mm remained for the duodenum. This resulted in closure of the duodenum. The right-sided portion of the duodenum expanded to 4 to over 5 cm. The high peristaltic pressure manifests itself in the fact that the contour of the duodenum rears up in a shoulder shape at the superior mesenteric artery (arrows). The stomach also has hardly any space (5.95 mm) in the median line, whereby the pancreas is compressed to 6.38 mm. (Such patients sometimes have impaired pancreatic function or slightly elevated lipase levels in the blood). The distance between the spine and the inner abdominal wall is only 2.16 cm, which points to the high degree of lordosis as the cause of this and the patient’s accompanying vascular compression syndromes (lordogenetic renal vein compression – often incorrectly referred to as “nutcracker syndrome” , May-Thurner syndrome and ligamentum arcuatum syndrome).
After feeding, the filling stomach pushed the superior mesenteric artery further to the right to a distance of 23 mm from the aortic axis. Now the filling stomach pushed the superior mesenteric artery to a minimal distance of 7.12 mm to the anterior edge of the lumbar vertebral body. A space of about 3 mm remained for the duodenum. This resulted in closure of the duodenum. The right-sided portion of the duodenum expanded to 4 to over 5 cm. The high peristaltic pressure manifests itself in the fact that the contour of the duodenum rears up in a shoulder shape at the superior mesenteric artery (arrows). The stomach also has hardly any space (5.95 mm) in the median line, whereby the pancreas is compressed to 6.38 mm. (Such patients sometimes have impaired pancreatic function or slightly elevated lipase levels in the blood). The distance between the spine and the inner abdominal wall is only 2.16 cm, which points to the high degree of lordosis as the cause of this and the patient’s accompanying vascular compression syndromes (lordogenetic renal vein compression – often incorrectly referred to as “nutcracker syndrome” , May-Thurner syndrome and ligamentum arcuatum syndrome).
 It is therefore not surprising that the aorta, which is often depicted in the middle of the spine in anatomical textbooks, has deviated 1.70 cm to the left of the midline. The superior mesenteric artery, on the other hand, has deviated 4.81 mm to the right of the midline. The situation can best be described as follows: the spine squeezes the upper abdominal organs against the inner abdominal wall, causing the arteries to deviate to the side, but the structures that run horizontally across the spine, such as the duodenum, pancreas and stomach, are highly compressed, which leads to an emptying disorder of the stomach and duodenum.
It is therefore not surprising that the aorta, which is often depicted in the middle of the spine in anatomical textbooks, has deviated 1.70 cm to the left of the midline. The superior mesenteric artery, on the other hand, has deviated 4.81 mm to the right of the midline. The situation can best be described as follows: the spine squeezes the upper abdominal organs against the inner abdominal wall, causing the arteries to deviate to the side, but the structures that run horizontally across the spine, such as the duodenum, pancreas and stomach, are highly compressed, which leads to an emptying disorder of the stomach and duodenum.
As long as the patient was fasting, the distance between the superior mesenteric artery (SMA) and the spine was 13.8 mm, whereby the artery had already deviated 5.3 mm to the right of the aortic axis (see following figure):
The superior mesenteric artery was displaced far to the right after feeding. The aorta was located on the left anterior curvature of the spine. Both vessels therefore described a wide angle in the horizontal plane.
In Wilkie syndrome and all other vascular compression syndromes in the abdominal cavity, increased lordosis of the lumbar spine (hollow back) plays a decisive role in the disease.
This was also the case in this patient. The pronounced forward curvature of the spine meant that there was only a gap of approx. 2 cm in front of the spine for the abdominal organs. As a result, the aorta, the stomach and the superior mesenteric artery did not have enough space to lie in a sagittal plane, as is usually depicted in standard anatomical textbooks.
The confined space had led to the duodenum being trapped between the superior intestinal artery and the spine instead of between the superior intestinal artery and the aorta, as is usually the case in Wilkie’s syndrome. The peristaltic wave was unable to elevate the superior iliac artery far enough against the abdominal wall to allow the food to pass through the horizontal portion of the duodenum unimpeded. The symptoms corresponded to those of a classic Wilkie syndrome.
It is important not to overlook those variants in which the classic anatomical constellation is not present, but in which the functional consequences and thus the clinical picture represent a considerable impairment for the patient. It is particularly impressive how the position of the upper abdominal organs changes due to the additional volume required by the filling stomach. This can produce completely different anatomical relationships than those usually seen on a fasting image.
For example, this fasting CT of the patient shows a completely different, “normal” topography of the ASM and aorta, namely in a sagittal plane:
This frequent observation once again emphasizes the need for a functional examination and close observation of the interaction of the upper abdominal organs during the functional test. Assuming an upright posture can also cause considerable changes in the position of the organs. It is not uncommon for the renal blood flow to be impaired after eating or while standing, with the increasing congestion of blood in the collateral circulation then triggering significant symptoms in the legs and pelvis during food intake and upright posture.
In the patient, the blood flow in both kidneys collapsed significantly when standing. However, even food intake causes a decrease in blood flow to the left and right kidneys when lying down.
 The following diagram shows that the peripheral blood flow in the right kidney drops from 53% in the fasting state to 44% after food intake. This is a clear indication of the increasing outflow resistance of the right renal vein, obviously due to its compression by the massively dilated duodenum.
The following diagram shows that the peripheral blood flow in the right kidney drops from 53% in the fasting state to 44% after food intake. This is a clear indication of the increasing outflow resistance of the right renal vein, obviously due to its compression by the massively dilated duodenum.
A quantitative assessment of the blood flow to the organs with the PixelFlux software, which was first developed 24 years ago by my son, Jakob Scholbach, is indispensable for the assessment of the manifold correlations of the disturbed organ function of various abdominal organs and provoking factors such as changes in body position and food intake.