- Neue Artikel
- Was Patienten sagen – Fortsetzung
- Praktiken/bokning av möten online
- Gefäßkompressionssyndrome
- Haben Sie Fragen?
- Checkliste Gefäßkompressionssyndrome
- Muskuloskelettale Besonderheiten der weiblichen Pubertät
- Lordose /Hohlkreuz – Ursache zahlreicher abdomineller Kompressionssyndrome
- Das ”Nussknacker”-Syndrom ist eine Fehlbezeichnung
- May-Thurner-Konstellation /May-Thurner-Syndrom/Cockett’s syndrome/Vena iliaca-Kompressionssyndrom
- Mittelliniensyndrom (Stauung der Mittellinienorgane)
- Pelvines Kongestionssyndrom
- Truncus-coeliacus-Kompression / Dunbar-Syndrom / MALS / Ligamentum arcuatum-Syndrom
- Wilkie-Syndrom / Arteria-mesenterica-superior-Syndrom
- Quantifizierung der Gefäßkompressionssyndrome mit der PixelFlux-Technik
- Bindegewebserkrankungen begünstigen kombinierte Kompressionssyndrome
- Pudendusneuralgie bei vaskulären Kompressionssyndromen
- Migräne und Multiple Sklerose
- Behandlung von Kompressionssyndromen
- Vaskulära kompressionssyndrom som jag nyligen upptäckt
- Kaleidoskop lehrreicher Krankheitsverläufe
- Ultraschalldiagnostik
- Leistungsspektrum
- Funktioneller Farbdoppler-Ultraschall – wie ich ihn verstehe
- Durchblutungsmessung – PixelFlux-Verfahren
- Forschung
- Publikationen
- Artiklar författade av Th. Scholbach
- Eigene Publikationen
- Erstbeschreibung der Bestimmung des Gewebsperfusionsindexes in Nierentranplantaten
- Erstbeschreibung des Mittellininesyndroms – Aspirintherapie
- Erste sonografische Gewebsperfusionsmessung in Nierentransplantaten
- Erste sonografische Tumorperfusionmessung und Korrelation zur Tumoroxygenierung
- Erstmalige Darmwandperfusionsmessung bei M. Crohn
- Erstmalige sonografische Gewebsperfusionmessung der Nieren
- Erstmaliger Nachweis von Frühveränderungen der Nierenperfusion bei Diabetes mellitus
- PixelFluxmessung der Nierengewebsperfusion
- Publikationen
- Expertise
- Bornavirusinfektion
- Wissenschaftliche Zusammenarbeit
- Cookie-Richtlinie
- Hinweise zu medizinischen Erläuterungen
- Datenschutzerklärung
- Cookie Policy (EU)

20 kg weight loss and unbearable abdominal pain and nausea after eating due to simultaneous compression of the celiac trunk and superior mesenteric artery.
The 59-year-old patient developed increasing unbearable epigastric abdominal pain after eating and severe postprandial nausea after undergoing fundoplication to correct a hiatal hernia in 2022. The abdominal pain is localised in the epigastrium and spreads as stabbing pain to the left flank. The patient reports nausea but does not vomit. Food intake is so severely restricted that the patient can only eat her first small meal in the afternoon. She also has a significant loss of appetite. As a result, she has lost 20 kg since 2022. In addition, she has persistent nuchal pressing headaches that increase to a severity of 10/10 after meals. This is accompanied by visual disturbances. Outside the hospital, Wilkie syndrome was suspected due to the narrow aorto-mesenteric angle. At the beginning of the fourth decade, the patient underwent a hysterectomy due to very heavy menstrual bleeding.
Clinical findings:
The patient reports persistent epigastric abdominal pain. Abdominal palpation intensifies this pain but also triggers localised pain in the lower left abdomen. No pathological resistance is palpable anywhere. However, the abdominal skin is noticeably soft. There are clear signs of hyperextensible joints with a Beighton score of 5/9, indicating hypomobility syndrome. The patient also confirms hypermobility in numerous joints and is able to clasp her hands behind her back, for example. The subcutaneous blood vessels of the abdominal skin are not dilated, there are no striae distensae, but there is noticeable hyperpigmentation of the abdominal skin. Auscultation of the abdomen reveals no vascular bruits and normal peristalsis.
Sonographic findings:
The urinary bladder is sufficiently filled, echo-free with a soft and smooth wall—the organ is therefore unremarkable on sonography. The distal ureters are not dilated. No free fluid can be detected in the pelvis.
The transverse diameter of the vaginal wall is 3 mm (normal up to 3 mm). The uterus is no longer detectable after hysterectomy. The vaginal stump is unremarkable, without cervix. Colour Doppler shows significant venous congestion of the urethra and, to a lesser extent, the vagina. Particularly strong twisting of the venous vessel is found between the urethra and symphysis. These are clear sonographic signs of pelvic congestion, which, however, the patient has apparently hardly noticed so far.
The maximum tissue perfusion of the pelvic organs (cm/s * cm² of the perfused area of the ROI /cm² of the ROI – normal < 0.05), measured under standardised conditions, is:
| urethra | 0,569 | 1138% |
| vagina | 0,029 | 58% |
The extent of pelvic congestion can also be expressed as the percentage of the left internal iliac vein involved in drainage of the pelvis—see the following table:
Venous drainage of both pelvic hemispheres is significantly reduced to ⅓ – ¼ of normal.
The left common iliac vein accumulates on the left flank of the sacrum to a sagittal diameter of 13 mm, with a maximum flow velocity of 16 cm/s. When crossing the elevated promontory, the vein is compressed dorsally, most strongly at the intersection with the right pelvic artery. Here, the sagittal diameter of the vein is reduced to 3 mm and turbulent flow acceleration to 204 cm/s occurs. This results in a significant venous outflow obstruction due to compression of the left pelvic vein—a May-Thurner constellation.
The outflow from the right pelvic hemisphere is also impaired because, as a result of the steep sacrum and significant forward tilt of the pelvis as an expression of increased lumbar lordosis, the origin of the vena cava is also pushed and compressed from the dorsal side, so that the sagittal vessel width is only 3 mm.
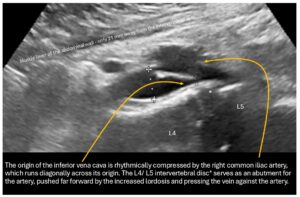 This leads to a significant acceleration of flow at the transition from the right common iliac vein to the vena cava from 29 to 76 cm/s.
This leads to a significant acceleration of flow at the transition from the right common iliac vein to the vena cava from 29 to 76 cm/s.
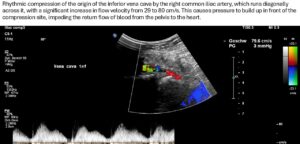 This creates an obstruction to drainage for both hemispheres of the pelvis, which particularly exacerbates the patient’s symptoms, as despite the absence of symptoms of pelvic congestion, the pelvic veins fail as collateral circulation of the congested left renal vein (described below).
This creates an obstruction to drainage for both hemispheres of the pelvis, which particularly exacerbates the patient’s symptoms, as despite the absence of symptoms of pelvic congestion, the pelvic veins fail as collateral circulation of the congested left renal vein (described below).
The increased lumbar lordosis manifests itself in the fact that the minimum distance between the front edge of the spine at the level of the L3/L4 intervertebral disc and the inner abdominal wall is only 20 mm. This is highly likely to lead to conflicts between the filling small intestine and the vessels in the vicinity, especially the veins, as the filling small intestine reaches a thickness of 20-25 mm. Therefore, a postprandial increase in compression of the vena cava and, if necessary, also of the pelvic veins is to be expected.
In addition, the right common iliac vein is rhythmically pushed against the promontory by the slightly elongated right common iliac artery, causing intermittent, almost complete collapse of the right pelvic vein at the pelvic outlet.
The further course of the vena cava is unaffected, with a sagittal width of 7 mm in its initial section, a width of 17 mm in the hepatic segment, and a width of 17 mm as it passes through the diaphragm. Flow velocities range from 52 cm/s at the diaphragm to 235 cm/s at the narrowest point of the vena cava.
As a result of the pronounced lordosis and the associated ventral displacement of the aorta, the left renal vein is also severely compressed.
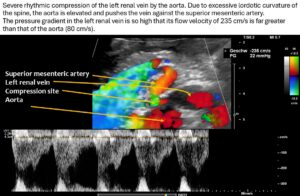 The narrowest point is located at the arcuate origin of the right renal artery. The lumen of the renal vein is barely recognizable. As a result, there is a significant acceleration of flow from 14 cm/s to the left of the aorta to 235 cm/s at the narrow point. This is a severe hemodynamically significant outflow obstruction of the left kidney, often somewhat misleadingly referred to as nutcracker syndrome. This constellation is responsible for the pain radiating from the epigastrium to the left hypochondrium. As the stomach fills, the compression exerted by the greater curvature on the tense renal vein intensifies the discomfort in the epigastrium. The left renal vein runs relatively far cranially.
The narrowest point is located at the arcuate origin of the right renal artery. The lumen of the renal vein is barely recognizable. As a result, there is a significant acceleration of flow from 14 cm/s to the left of the aorta to 235 cm/s at the narrow point. This is a severe hemodynamically significant outflow obstruction of the left kidney, often somewhat misleadingly referred to as nutcracker syndrome. This constellation is responsible for the pain radiating from the epigastrium to the left hypochondrium. As the stomach fills, the compression exerted by the greater curvature on the tense renal vein intensifies the discomfort in the epigastrium. The left renal vein runs relatively far cranially.
A collateral circulation branches off from the left renal vein to the spinal canal—a so-called tronc réno-rachidièn—which feeds 148 ml/min into the epidural plexus in a horizontal body position. When standing, the perfusion of the vessel increases to 531 ml/min.
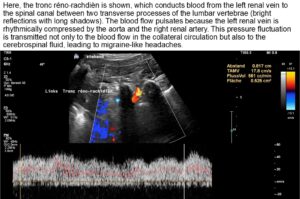 This contributes to an increase in pressure in the spinal canal and subsequently intracranially, which would explain headaches and, in some cases, neurological deficits in the cranial nerves (visual disturbances, speech disorders, bulbar motility disorder). The drastic increase in spinal and thus cerebral congestion when standing after eating. This corresponds exactly with the patient’s description of unbearable headaches after every meal.
This contributes to an increase in pressure in the spinal canal and subsequently intracranially, which would explain headaches and, in some cases, neurological deficits in the cranial nerves (visual disturbances, speech disorders, bulbar motility disorder). The drastic increase in spinal and thus cerebral congestion when standing after eating. This corresponds exactly with the patient’s description of unbearable headaches after every meal.
The systolic flow velocity in the abdominal aorta is only 80 cm/s. This indicates the compliance of the aortic vessel wall. However, in the celiac trunk, there are significantly increased flow velocities that vary with breathing, namely 161 cm/s in the middle respiratory position, 287 cm/s during inspiration, and 210 cm/s during expiration. This indicates ligamentum arcuatum syndrome, whose typical symptoms are vegetative symptoms such as nausea, circulatory dysregulation, disturbed peristalsis, sudden skin redness, and loss of appetite, as well as, of course, severe postprandial epigastric abdominal pain—the patient’s main symptom.
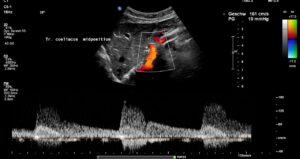
The arcuate ligament pushes the celiac trunk so far caudally that it leads to secondary compression of the superior mesenteric artery.
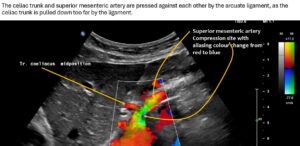 As a result of the compression, turbulent, highly accelerated flows are found in the vessel outlet, with 240 cm/s in the middle respiratory position, 226 cm/s during inspiration, and 196 cm/s during expiration.
As a result of the compression, turbulent, highly accelerated flows are found in the vessel outlet, with 240 cm/s in the middle respiratory position, 226 cm/s during inspiration, and 196 cm/s during expiration.
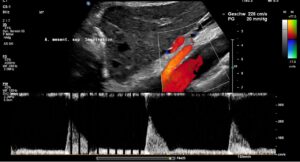
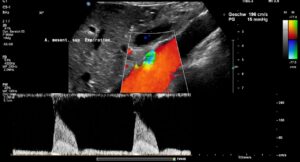 Since both upper abdominal arteries are compressed, there is a risk of intestinal ischemia.
Since both upper abdominal arteries are compressed, there is a risk of intestinal ischemia.
The splenic vein and superior mesenteric vein are unaffected.
Both kidneys have a strong, homogeneous, hypoechoic parenchyma and no signs of impaired urine transport. The kidney volumes are 64 ml/m² on the left and 58 ml/m² on the right. The 50th percentile for normal kidney volume is 62 mL/m², while the upper limit of the normal range is 82 mL/m².
The significant orthostatic nephroptosis on the right was calculated from the change in distance between the kidney and the iliac crest when changing body position and is shown in the table below:
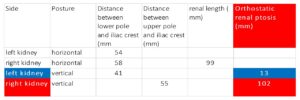
To determine the extent of orthostatic venous pooling in the pelvis and legs, the perfusion volume of the abdominal aorta at the level of the aortic hiatus was determined in the supine position and in the upright position:
When standing, there is a 44% reduction in filling of the left ventricle as a result of additional orthostatic venous pooling in the pelvis. The only moderate response of the heart rate cannot therefore prevent the circulating blood volume of the aorta from decreasing by 40% when standing. This explains weakness, cerebral underperformance due to cerebral underperfusion, and possibly even presyncope or syncope.
The PixelFlux measurement of blood flow in the renal parenchyma in the following diagrams shows a clear reduction in perfusion of the left kidney already when fasting in a lying position and postprandially when standing. Only when lying down postprandially is there a reduction in blood flow to the right kidney. This may be an expression of the mechanical defect of the filling duodenum on the right renal vein.
Diagram of perfusion measurement of the renal parenchyma using the PixelFlux technique
The columns show the perfusion intensity in cm/s, calculated as the perfusion velocity [cm/s] of all colored pixels multiplied by the area of all colored pixels [cm²] divided by the area of all pixels in the entire region of interest [cm²].
 After consuming a test meal, the pars horizontalis duodeni between the aorta and the superior mesenteric artery expands from 2 mm on an empty stomach to approximately 10 mm. There are no peristaltic-synchronous complaints, although not every peristaltic wave transports intestinal contents across the aorta. However, this rules out Wilkie syndrome.
After consuming a test meal, the pars horizontalis duodeni between the aorta and the superior mesenteric artery expands from 2 mm on an empty stomach to approximately 10 mm. There are no peristaltic-synchronous complaints, although not every peristaltic wave transports intestinal contents across the aorta. However, this rules out Wilkie syndrome.
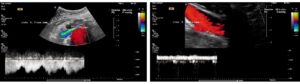
The drastic difference in diameter between the much smaller superior mesenteric artery and the superior mesenteric vein is immediately apparent. This is the reason for measuring the volume flow in both vessels using the 4D-PixelFlux technique.
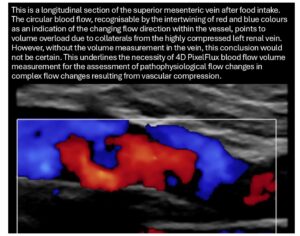
The result clearly shows that the flow volume of the vein is within the expected range, whereas this is not the case for the arterial flow. This could be due to the combined effect of compression of the superior mesenteric artery, which is not supported by the celiac trunk, which is also compressed. On the other hand, this discrepancy could be exacerbated by the possible inflow of venous blood from the area of the compressed left renal vein and left common iliac vein—the so-called Retzius collaterals. The following image shows the overload of the transport capacity of the superior mesenteric vein—due to the overfilling of the vessel, the blood cannot flow out without resistance but instead circulates partially within the vessel.
Diagnoses:
- Hypermobility syndrome
- Increased lumbar lordosis as a result of connective tissue weakness, which allows hypermobility
- Pelvic congestion in a patient with hysterectomy 23 years ago
- Severe compression of the left common iliac vein at the junction with the right pelvic artery – May-Thurner syndrome
- Compression of the caudal portion of the vena cava due to increased lumbar lordosis
- Extreme lordogenic left renal vein compression (see also here and here)
- Collateralization of renal vein blood, including into the spinal canal via a tronc réno-rachidièn, resulting in increased postprandial spinal and cerebral congestion
- Ligamentum arcuatum syndrome (MALS)
- No Wilkie syndrome
- Condition after fundoplication, whereby an increase in pressure in the upper abdomen due to difficult emptying of the stomach appears to be a possible trigger for the symptoms after the operation
- Significant orthostatic nephroptosis on the right, which, however, has no adverse effect on renal blood flow
- Signs of possible collateralization of venous blood from the compressed left renal vein toward the mesenteric circulation
Recommendation:
I recommend surgical decompression of the left renal vein and left pelvic vein by encasing them in a PTFE sleeve and excision of the ligamentum arcuatum and scarred parts of the celiac plexus. After decompression of the celiac trunk, spontaneous decompression of the superior mesenteric artery is expecter to occur.