Stent failure in abdominal venous compression syndromes
2019-08-24 and continued
1: Stent increases other vascular compression
2: Travelling stent producing new compression
4: Reoccurrence of former symptoms plus new ones
5: Two overlapping stents collapsing while upright, open while laying supine
6: Threefold collapsed stent causes postprandial compression of the splenic vein
7: Gravitational stent dysfunction
Example 1: Stent increases other vascular compression
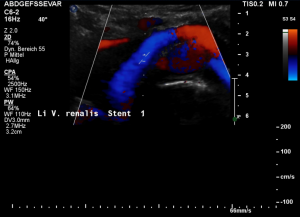
Stenting of the left renal vein
Harmful additional compression of the celiac trunk by a stent within the left renal vein
The main problem, the coexisting May Thurner syndrome, was missed despite a CT of the abdomen
Always search for additional compressions
Example 2: Travelling stent producing new compression
Dislodgement of a stent being too short
Example 3: Intruding painful stent
Mishappened stenting of the left common iliac vein to treat May-Thurner syndrome
Pain in the pelvic area because of a still relevant venous congestion of the uterus
Why is the uterus still congested despite „successful “stenting of the left common iliac vein?
Why does the patient suffer so immense pain in a small area in her left lower abdomen?
Example 4: Reoccurrence of former symptoms plus new ones
The stent slipped out of the compression site
Example 5: Two overlapping stents collapsing while upright, open while laying supine
Two overlapping stents had been set
Changing of body position causes collapse of the stent
Another important vascular compression was left undetected prior to stenting
Example 6: Threefold collapsed stent causes postprandial compression of the splenic vein
Proof of the functional insufficiency of the collapsed stent by the PixelFlux measurements
Postprandial compression of the splenic vein by the left renal venous stent
Example 7: Gravitational stent dysfunction
The stent squeezing the right renal artery
The stent squeezes the splenic vein too-but only while standing upright
Further vascular compression-so far undetected
Compression of the vena cava inferior by bowel loops
A May-Thurner syndrome was left undetected so far
Example 9: A stent reducing one compression – creating three new ones!
Stent failure in abdominal venous compression syndromes
by Thomas Scholbach / 2019-08-24 ….
www.scholbach.de / praxis.scholbach@posteo.de
Example 1: Stent increases other vascular compression
The patient started suffering 5 years ago with increasing intensity of abdominal pain around her umbilicus. She did not lose weight and keeps her weight at around 50 kg. Her actual weight is 49.5 kg, her actual height is 163 cm.
An abdominal CT was carried out which led to a diagnosis of a nutcracker syndrome and a Wilkie syndrome. While the nutcracker syndrome was treated with a stent insertion into the left renal vein in November 2017 and an embolization of the left ovarian vein, the Wilkie syndrome was left untreated since the aorto-mesenteric angle was apparently wide enough after the insertion of the renal venous stent.
Unfortunately, the insertion of the stent did not change her symptoms. Despite of the treatment, her pain even increased by 1/10 and is extreme during menstruation with a degree of 8-9/10 but is also felt constantly with a degree of 8/10 all the time in the periumbilical area. There are rarely episodes of diarrhea, not related to the pain intensity. The patient has lost her strength, cannot stand or walk longer than 3 hours. The pain also increases after a meal, starting around 3 hours later. Swelling of her legs, a disturbed breathing or fainting are denied. After longer physical activities she feels pain in the middle of her lumbar spine.
Stenting of the left renal vein
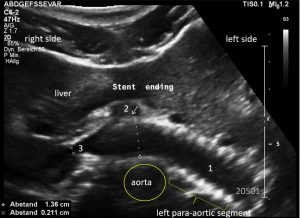
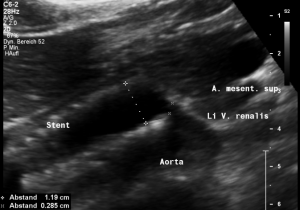
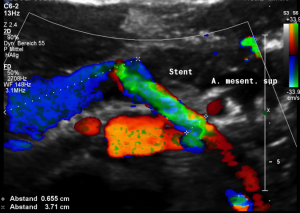
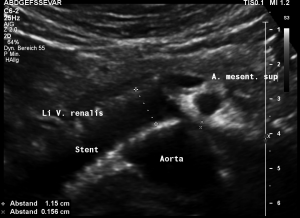
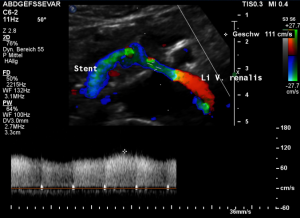
This stent [1] reaches to less than the half of the superior mesenteric artery [2].
This poses the stent at risk to flip back into the left para-aortic segment of the left renal vein, thus letting the superior mesenteric artery compressing the left renal vein again.
Already now, a narrowing [3] to 2 mm is found before the entering of the left renal vein into the vena cava inferior.
Thus, the stent is already dysfunctional and may become functionless, if only a slight movement of about 4 mm towards the left side occurs.
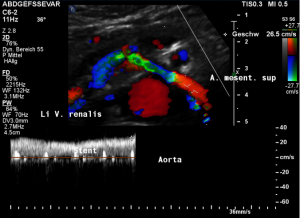
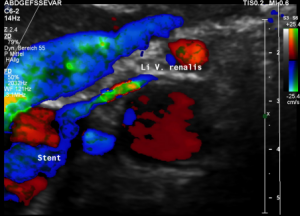
Harmful additional compression of the celiac trunk by a stent within the left renal vein
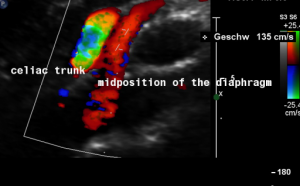
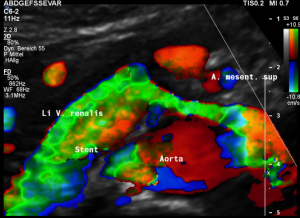
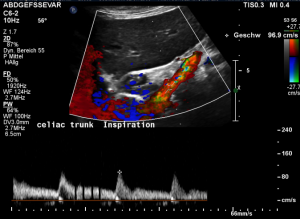
This is a sagittal transection of the stent within the left renal vein. The SMA is depicted in red, the celiac trunk shows a sharp flow acceleration, evident by the localized aliasing (discoloration of blood flow from red to blue). This points to the fact, that the stent is pressing the SMA against the celiac artery which in turn is compressed from above by the arcuate ligament.
So, in this situation, the stent increases her complains which are caused by a median arcuate ligament syndrome by adding pressure against the celiac artery from below.
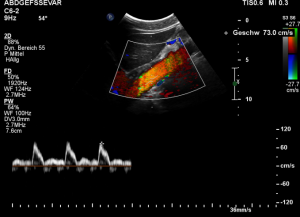
Demonstration of the additional compression of the celiac trunk by comparing its flow velocity with the flow velocity in the aorta
Within the celiac trunk the flow velocity is 161 cm/s, doubling the flow velocity of the aorta, thus pointing to a relevant compression of the celiac trunk.
Summary
The stent within the left renal vein is too short, to dilate the compression of the vessel completely. A narrowing is left untreated at the mouth with the vena cava inferior.
Moreover, the stent increases the compression of the pre-existing but so far undiagnosed celiac artery compression syndrome (MALS).
The prior examinations also missed the existence of a severe May Thurner syndrome which caused the main symptoms of the patient. Her pain was localized at the umbilicus, exactly the position, where in her case the left common iliac vein was compressed by the overriding right common iliac artery.
The main problem, the coexisting May Thurner syndrome, was missed despite a CT of the abdomen
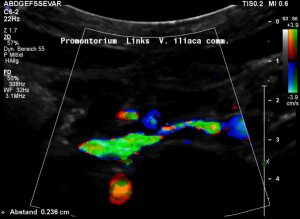
Grossly dilated left common iliac vein before ascending the left lateral slope of the sacral bone. Its diameter is 15 mm.
Compression of the left common iliac vein underneath the right common iliac artery. Its diameter is 2 mm.
High-grade compression of the left common iliac vein causes pain at the compression site and pelvic congestion with pain in the depth of the pelvis
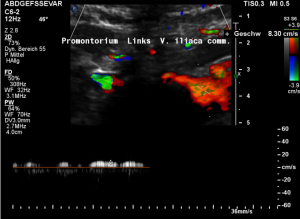
Low flow velocity of 8 cm/s within the dilated segment of the left common iliac vein.
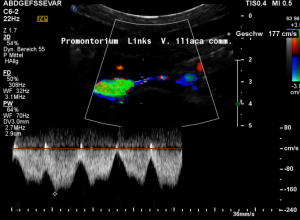
Rapid flow acceleration to 177 cm/s within the compressed segment of left common iliac vein.
Conclusion
The very reason for the patient ‘s suffering, the May-Thurner syndrome was missed in prior CT examinations. It is responsible for the periumbilical unbearable pain in this patient.
The coexisting left renal vein compression was treated unsuccessfully by a venous stent.
This stent not only failed to open the left renal vein compression completely but caused an additional deterioration of the coexisting median arcuate ligament syndrome, which was also undiagnosed in the CT of the abdomen.
Always search for additional compressions
If one compression syndrome is found, all other compression syndromes must be ruled out, since they usually run together. One compression syndrome rarely comes alone!
The stenting of the left renal vein did not treat the source of the periumbilical pain, which consequently persisted after the stenting. The patient was then told, that her persistent suffering „was due to a psychological problem“.
Moreover, the stenting was harmful to the pre-existing but also undiagnosed median arcuate ligament syndrome.
Example 2: Travelling stent producing new compression
Her symptoms started in March 2018. From then on, she suffered from severe and increasing abdominal pain in the epigastric angle. In September 2018 she began to vomit continuously and could not even take in fluids. Then the diagnosis of a Wilkie- syndrome was made and a duodeno-jejunostomy was carried out in October 2018.
After this operation some improvement was achieved in terms of less vomiting. She was then even able to take some small bites of solid food by mouth.
Later, the vomiting reoccurred. Since pain in the left renal fossa developed now, a diagnosis of a left renal vein compression was made, and a stent was inserted into the left renal vein in February 2019.
Unfortunately, this had no effect on the severe pain in her left flank. Today she arrived in my clinic, vomiting for 14 hours and collapsing on arrival. She fainted several times due to terrible pain.
Clinical examination
Her physical examination reveals strong pain on palpation in the epigastric angle as well as in the left hypochondrium and to a lesser extent above both ovaries.
During the examination, a very specific and narrowly confined area with a diameter of about 4 cm, located about 6 cm cranially to the umbilicus and 3 cm paramedial to the right, can be identified as a source of extreme pain which does not allow touching – otherwise, the patient collapses.
There is an area of less severe painful sensation in the left hypochondrium.
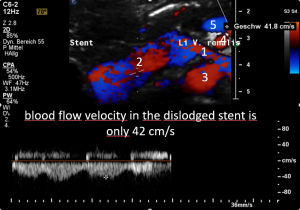
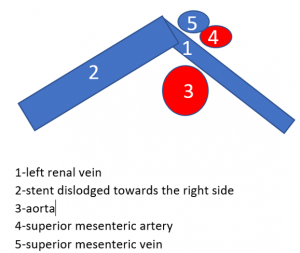
Dislodgement of a stent being too short
The stent, intended to hold apart the superior mesenteric artery and the aorta, slipped out of this clamp towards the right side. Therefore, the patient is in pain again, suffers renal bleeding, the stent pokes into the wall of the left renal vein, posing it at risk to penetrate the wall. Moreover, the stent may travel further into the vena cava and from there into the heart which would be a life-threatening development.
Summary
In some patients the stent is chosen too short. Already after a few weeks the stent is moving, most likely towards the right side, driven by the blood flow towards the vena cava inferior. The reason for the high mobility of venous stents in vascular compression syndromes is the fact that there is a high local pressure onto the stent at the compression site. The compression may be exerted by the stiff aorta, as in lordogenetic left renal vein compression, or by the bony spine itself, as in May Thurner syndrome.
Venous abdominal compression syndromes are substantially different from other disorders, treated by a stent. In contrast to arterial stents, venous stents are more rigid than the vessel wall itself. This is the reason for malalignment of the stent and the vessel. So, it is often observed, that in the curved course of compressed veins the stent spans out the vein, thus causing an even further stretching. Since/stretching of the vein was the primary because of its compression, stents in venous compression syndromes often alleviate one compression and causing a new one, most frequently at the proximal opening of the stent.
Example 3: Intruding painful stent
The patient was diagnosed with a triple compression, left renal vein compression, left common iliac vein compression and the celiac trunk compression.
In October 2018 a stent was inserted into the left common iliac vein to treat a May-Thurner constellation.
Two attempts, to treat a lordogenetic compression of the left renal vein with a stent failed. The first time the stent could not be placed, the second time it dislodged during the procedure and had to be retrieved from the heart via the right internal jugular vein, the largest vein in the neck.
She suffers now from severe nausea and vomiting, gulping of gastric acid and constipation. Her left upper thigh is painful, she develops hypotonic attacks with tachycardia. Recently a strong pain in her both flanks emerged.
Mishappened stenting of the left common iliac vein to treat May-Thurner syndrome
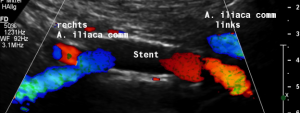
The stent does not bridge the May-Thurner point [1].
Therefore, the compression of the left common iliac vein persists
This stent has slipped towards the left side of the compression point [1], thus reinstituting the venous compression which was meant to be relieved by the stent.
Moreover, the leftward shifting of the stent now causes the stent to poke into the curved proximal left common iliac vein, thus causing severe pain exactly at the site of spearing into the venous wall (to be seen in the following images).
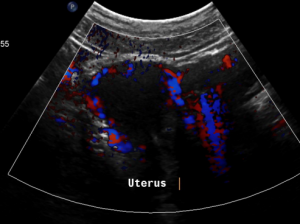
Pain in the pelvic area because of a still relevant venous congestion of the uterus
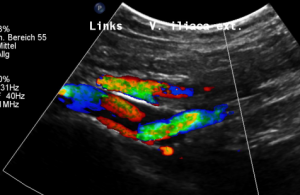
The myometrium [1] of the uterus is filled up with winding veins [2], trying to transport blood from the congested left pelvic axis towards the right pelvic axis via midline organs as the uterus (usually the major route), the urinary bladder, the vagina, and the urethra.
Why is the uterus still congested despite „successful “stenting of the left common iliac vein?
Pelvic congestion syndrome, mainly affecting the uterus and the deep veins inside the pelvis, consists of an overload of the veins inside the uterus or outside the uterus in the deep pelvis.
The accumulation of the venous blood has two possible reasons:
Obstruction of the venous outflow
Volume overload due to collaterals from other organs, mainly the left kidney
In this patient the left renal vein compression was left untreated
A stent was placed into the left common iliac vein to treat a coexisting May Thurner syndrome
The stent developed a malalignment with the curving left common iliac vein thus causing a poking pain at its proximal opening, now the main problem for the patient. In addition, despite the patent lumen of the stent, its proximal opening was partially obstructed by the left common iliac vein which hang down in a tent-like manner, thus partially obliterating the opening of the stent.
Due to this stent obstruction at its proximal opening, the capacity to transport the additional venous blood, running down through the left ovarian vein from the highly pressurized left renal vein, was too low.
Thus, despite placing a stent which is still open, the stent is functionally insufficient to alleviate the congestion of the patient.
Why does the patient suffer so immense pain in a small area in her left lower abdomen?
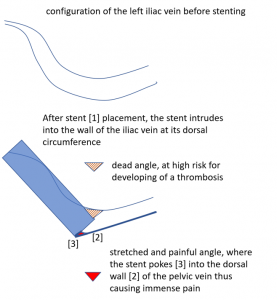
The stent is not in line with the curved position of the left pelvic veins – the left external iliac and the left common iliac vein, the latter being the continuation of the first one.
Thus, the proximal opening of the stent spears into the dorsal wall of the left external iliac vein.
This causes a massive stretching as well as a chronic inflammation at the contact point of the stent with the dorsal wall of the vein
Exactly here, proven by one finger palpation under sonographic observation, the pain can be located.
So, the stent, trying to solve the compression, poses the patient at risk for a painful venous damage.
Moreover, in this patient, the stent failed to work properly, since it slipped out of the compression site towards the left, leaving the compression in place, which was meant to be treated by the stent.
In this case, the stent did harm instead of help
Also, the left renal vein compression was left untreated, which fuels the volume overload of the insufficient stent.
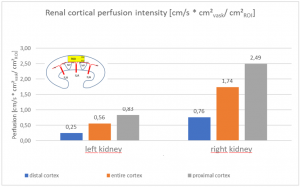
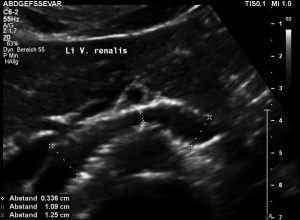
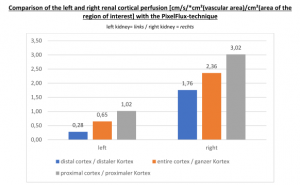
The insufficiency of the collateral circulation of the still compressed left renal vein is measured with the PixelFlux technique, demonstrating the high-grade perfusion loss of the left kidney- shown in the next image
The untreated left renal vein compression is the reason for a substantially reduced perfusion of the left kidney
PixelFlux measurements of renal parenchymal perfusion clearly quantify the loss on the left side
Summary
Lordogenetic left renal vein compression and compression of the left common iliac vein are a functional unit. They influence each other, since the blood can move through the left ovarian vein from one vessel to the other.
The impact of both compressions must be quantified, preferably by the PixelFlux technique, to evaluate the contribution of each compression syndrome to the clinical complaints of the patient.
In this patient two therapeutic errors can be demonstrated:
The left renal vein compression was left untreated, thus forcing large amounts of renal blood to enter the pelvic circulation. To stay without complaints in such a situation requires a widely open left common iliac vein.
The attempt to open the compressed left common iliac vein by inserting a stent failed here. The stent slipped towards the left, leaving the compression untreated. Moreover, the slipping produces the risk of penetration of the proximal stent opening into the dorsal wall of the left external iliac vein. This causes much pain and the risk for thrombosis at the opposite circumference of the stent ‘s entrance.
Moreover, the therapeutic goal to restore a normal blood flow capacity of the left common iliac vein was not attained.
Example 4: Reoccurrence of former symptoms plus new ones
This patient was diagnosed with a nutcracker syndrome in 2017 because of a persistent hematuria and pain in her left flank as well as in her left lower abdomen. The pain was so intense that she woke up in the morning and had to see the emergency department several times. In April 2019 a stent was placed into the left renal vein which led to some improvement, mainly of her flank pain and dizziness. On the other hand, other symptoms emerged or got worse as gastro-esophageal reflux, pain in the epigastric area and around her navel and difficulties with inspiration.
A gastroscopic examination came out normal.
Now, 2 months after the insertion of the stent, the original symptoms are back again in addition to pain in her left arm, left neck left and face, pulsating headaches on the left side of her skull and pain in her left leg. Also, hematuria returned. No vomiting and no diarrhea occurred. She now feels as if a wave of heat would emerge from her mid abdomen to ascend to her upper abdomen. The occurrence of near fainting and blood pressure instabilities is demanding a lot from the patient.
The patient cannot stand for longer times because of her pain and general weakness.
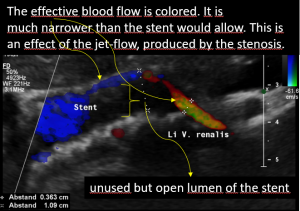
The stent slipped out of the compression site
This stent moved away from the original compression site towards the right of the aorta and superior mesenteric artery.
That is why the compression returned and the stent has lost its function.
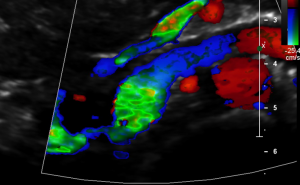
The turbulent flow, depicted in green and yellow, points to a sharp flow acceleration as a sign of a severe compression.
Compare the flow velocities within the compression site, depicted in the sonogram above, with the much lower flow velocity in the image before where the measurement probe is situated in the left para-aortic segment of the left renal vein which is left to the compression. Flow velocity jumps from 26 to 111 cm/s due to the re-compression.
Closeup view of the relevant structures and excellent depiction of the high flow turbulences depicted in green.
Not only the stenting was inadequate, but another relevant compression syndrome was missed-now causing additional problems
The patient also had a celiac artery compression syndrome which was aggravated by the stent insertion.
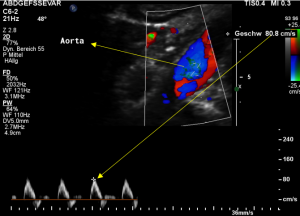
The flow velocity within the aorta was only 73 cm/s which is much lower than the flow velocity within the compressed celiac trunk.
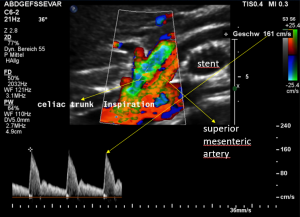
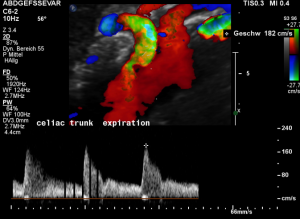
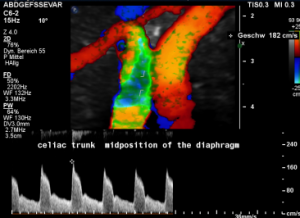
For image clearly shows a boomerang-like deformation of the celiac trunk by the straddling arcuate ligament, compressing the artery from above. This compression causes a flow acceleration to 182 cm/s. This finding is most prominent in the expiratory position of the diaphragm.
In inspiration, the velocity drops (97 cm/s) into a range similar to the aortic flow velocity since to arcuate ligament moves away from the vessel thus reducing its compression.
The image above shows the massive positional change of the celiac trunk during inspiration compared with expiration. The boomerang-like deformation completely disappears, and the artery now runs in a straight manner, its flow velocity drops significantly.
Schematic explanation of the harmful coexistence of celiac artery compression and left renal vein compression
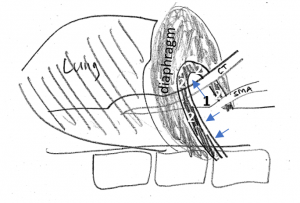
This sketch shows the relevant anatomic structures in inspiration. The diaphragm is contracted to descend towards the abdomen. The lungs are filled with air and the aortic hiatus [1], resembling the entrance into a tunnel, being surrounded by the arcuate ligament [2], takes a more horizontal direction.
So, the celiac trunk [CT] can run uncompressed.
In expiration, the diaphragm relaxes, thus assuming a more cephalad position. This way, the lungs are compressed to exhale. The aortic hiatus is now in a more vertical position, approaching the spinal column. Thus, the space for the passage of the celiac trunk is significantly reduced. The arcuate ligament tightly compresses the celiac trunk and pulls it down in a boomerang-like fashion.
If the diagnosis of a celiac artery compression is missed in patients with a compression of the left renal vein, then the insertion of a stent may be harmful to the pre-existing pressure onto the celiac trunk.
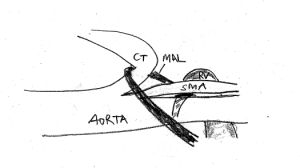
This sketch demonstrates the coexistence of celiac artery [CT] compression and left renal vein [LRV] compression. The median arcuate ligament [MAL] pulls down the celiac trunk and adds to the compression of the left renal vein. The celiac trunk sits tightly on the origin of the superior mesenteric artery, thus preventing the opening of the aorto-mesenteric angle, the natural passageway for the left renal vein.
If in such a situation a stent forces the SMA cephalad, it exerts additional pressure onto the celiac trunk from below.
This sketch shows the more pronounced celiac trunk compression after lifting the SMA to open the aorto-mesenteric angle for a better drainage of the left renal vein.
Conclusion
It is therefore crucial to rule out all possible vascular compression syndromes before deciding on a treatment.
If not all facets the lordogenetic vascular compressions* are known, a treatment is risky, may be harmful and may induce irreversible damage to the treated structures.
Not rarely, harmful stents have to be removed in order to relieve the symptoms of the patient. This can often be achieved only by replacing the stented vein by a graft.
*Non-exhaustive list of lordogenetic compressions
- Celiac artery compression
- Left renal vein compression
- Duodenal compression
- Left common iliac vein compression
- Femoral vein compression
- Splenic vein compression
- Vena cava inferior compression
- Renal artery compression
- Lumbar artery compression
- Stomach compression
Example 5: Two overlapping stents collapsing while upright, open while lying supine
Development of complaints
In 2014, during pregnancy a strong pain developed in the epigastric angle as well as in the left hypochondrium. After delivery, the pain persisted and grew in terms of extension and severity.
The actual symptoms consist of a constant pain in the line between the umbilicus and the epigastric angle and slightly off the midline towards the left hypochondrium and then in a larger area below the left rib cage towards the left flank. The pain is accompanied by lumbar pain and shortness of breath, pain and pressure in the left hemisphere of the face and a pulse-synchronous tinnitus in the left ear. She cannot tolerate lying on the left side because then tachycardia and pain develop.
Since pain is also aggravated by the larger meals you can tolerate only small portions and must eat nearly continuously all day over. In bouts of pain attacks the severity of pain is 8/10 and may last with this intensity for 1 week.
In 2016 the diagnosis of a nutcracker syndrome was made, and 2 stents have been placed into the left renal vein. After this intervention the flank pain decreased but the epigastric pain increased a lot. She now feels weak and has a constant tenderness in the left hypochondrium. Her left arm and left leg have a lost their strength.
After the stent insertion another surgery was carried out to correct a median arcuate ligament syndrome.
Since then she had more problems with inhaling, experienced an early satiety but had never vomited nor developed a diarrhea.
All these symptoms increased over the years and are now barely bearable, being most severe while standing, so that standing for a longer time is a real burden.
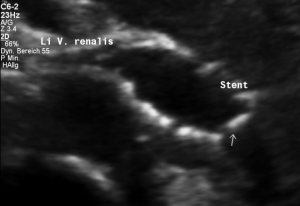
Two overlapping stents had been set
An undisturbed blood flow is seen through both overlapping stents. The lumen of both stents fit to each other and hold the former compression site open.
In a detailed review, however, it can be seen, that at their ends both stents to not align. The stent from the right side does not continue on its dorsal circumference with the stent on the left side. This weakens the entire structure.
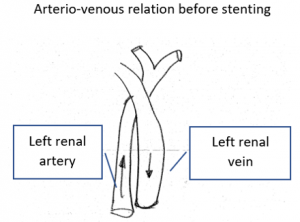
Body posture matters a lot
So far, the examination was carried out in a supine position of the patient, the same position which the patient assumes in CT or MRI devices.
Open stent but disturbed flow
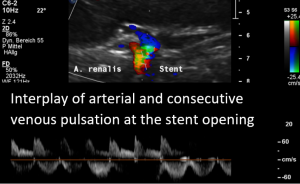
In the supine position a disturbed blood flow was found within the stent.A normal peripheral venous blood flow is a steady one with little variation only.
Despite the stent was open a disturbed pulsating flow pattern within the stent was eye-catching:
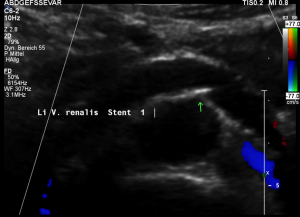
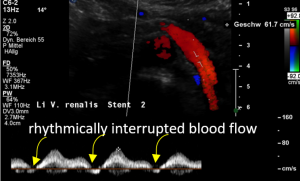
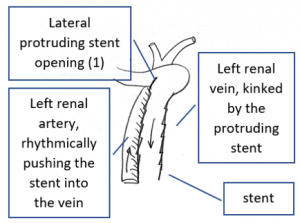
- Within the left side part of the stent a rhythmically interrupted blood flow was found.
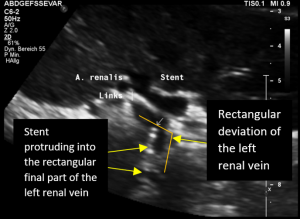
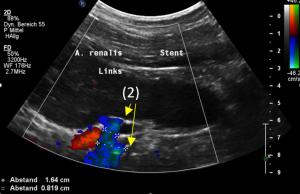
The reason for it is an intermittent obstruction of the blood flow with a pulsatile compression of the left lateral stent opening (1) by the overlapping left renal artery which pushed the stent into the free part of the left renal vein which deviated from the course of the stent in a rectangular fashion.
The stent opening is partially occluded (2) by the adherent left renal artery
While opening the primary compression in front of the aorta the stent causes a new obstruction at its left lateral origin.
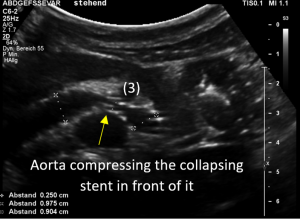
Changing of body position causes collapse of the stent
In the supine position the stent at least was open and allowed a relevant drainage of the left kidney. But the patient had reported that she could not stand upright since then the abdominal pain became unbearable.
So, the examination was repeated in a standing position.
Immediately after standing upright, the lordosis increased, thus pushing the aorta ventrally towards the abdominal wall. The stent cannot stand this pressure and collapses. Now it becomes obvious, that not only one stent was inserted but two stents which overlapped exactly in front of the aorta. This construction had its foreseeable breaking point (3) exactly in this region.
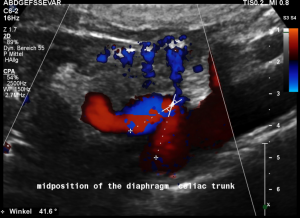
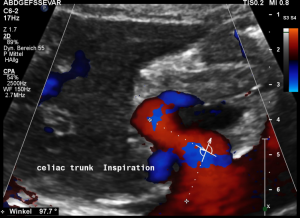
Another important vascular compression was left undetected prior to stenting
Some of the patient’s complaints are characteristic for a median arcuate ligament syndrome. Color Doppler ultrasound revealed a folding of the celiac trunk’s origin.
In midposition of the diaphragm the median arcuate ligament (4) compresses the celiac trunk from above. During inspiration the ligament retracts, and the trunk unfolds.
The effect of the compression can be quantified by measuring the angle formed by the compressed celiac trunk. In this case it increases from 42° to 98°, thus proving the dynamic nature of the compression by the diaphragm.
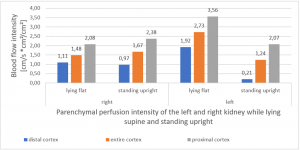
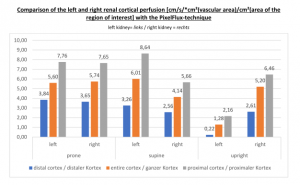
PixelFlux measurements clearly show the detrimental effect of the collapsing stent onto the blood flow of the left kidney
Without a sensitive method to measure the blood flow in the affected organs it is not possible to come to a clear conclusion concerning the effect of a vascular disorder.
PixelFlux is nowadays the first and only sonographic measurement technique to calculate exactly flow intensities and flow volume is in organs and tissues by means of routine color Doppler ultrasound. The diagram below shows the tremendous effect of the collapsing stent. In an upright position the left renal parenchymal perfusion of the subcapsular vessels drops from 1.9 to 0.21 cm/s *cm² (perfusion area)/cm² (total parenchymal area). This exactly reflects the unbearable situation which was described by the patient but was neglected by the treating physicians since they could not demonstrate any harmful effect of the stent with the
available standard imaging procedures.
Conclusion
The sophisticated color Doppler sonographic examination of all relevant vessels in a patient with one lordogenetic compression is ventilatory for a treatment success.
In this case the median arcuate ligament syndrome had been missed. Thus, its symptoms persisted after the stenting procedure.
The treatment of the lordogenetic left renal vein compression, erroneously named Nutcracker syndrome, with 2 slightly overlapping stents completely failed.
At the overlapping area a foreseeable breaking point was created.
The left of both stents moved laterally by the rhythmical compression of the adjacent left renal artery.
This rhythmic pressure protruded the left stent into the portion of the left renal vein.
The stent thus caused a kinking and partial obstruction of the left renal vein.
Thus, the function of the stent was compromised already in a resting supine position.
Moreover, in upright position caused an unbearable pain for the patient.
The patient was not trusted by her treating physicians and all her suffering was thought to be psychogenic.
This led to despair and hopelessness.
Standard imaging procedures as CT are unable to detect such types of stent dysfunction.
Only a thorough functional color Doppler sonography with a PixelFlux measurements in different body posture can detect the real source of the patient’s suffering in such a situation.
Example 6: Threefold collapsed stent causes postprandial compression of the splenic vein
History
The patient suffered from 2015 with daily diarrhoea, frequent urination and a constant feeling of urgency, pain and burning sensation of the bladder, severe menstrual pain, constant pelvic pain and progressive weight loss of 7 kg within six months. In 2017 a pelvic congestion was diagnosed by CT and the left ovarian vein was embolized. Nevertheless, the severity of menstrual pain was unchanged.
Then a nutcracker syndrome was diagnosed and in 2018 a stent was placed into the left renal vein which produced an even stronger stabbing and continuous pain under the left rib cage extending towards the back and towards the left kidney. The menstrual pain is disabling, extremely strong. The patient is now tired on a daily basis. She suffers from tachycardia after minimal effort and constant nausea without vomiting.
She cannot eat properly because of the pain.
The threefold collapsed stent
The stent had been collapsed on both ends and in its center.
Collapse of the central part of the stent
Turbulent flow acceleration of the collapsed right sided opening of the stent
Collapse of the left sided opening
Proof of the functional insufficiency of the collapsed stent by the PixelFlux measurements
The PixelFlux measurements clearly demonstrate the functional insufficiency of the collapsed stent. On the left side the suppressed parenchymal perfusion of the left kidney is depicted. The blood flow loss is most pronounced in the subcapsular vessels (blue) where only 16% of the perfusion of the right kidney can be measured. These subcapsular blood vessels are the tiniest ones and are thus compressed first.
Missed MALS
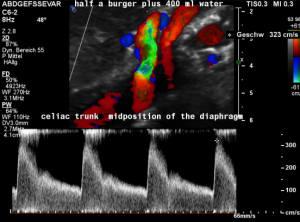
A coexisting median arcuate ligament syndrome was left undetected.
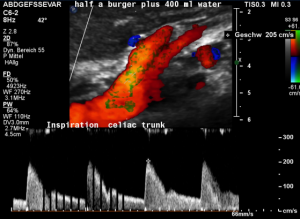
In midposition of the diaphragm a circumscribed compression of the origin of the celiac trunk is highlighted in green with a corresponding flow acceleration up to 323 cm/s!
In inspiration, after retraction of the arcuate ligament, the vessel widens and runs in a straight fashion with a reduced flow acceleration to 205 cm/s.
These are the typical sonographic signs of a median arcuate ligament syndrome
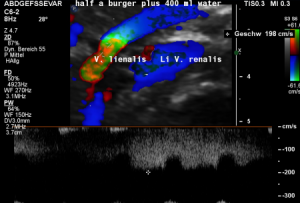
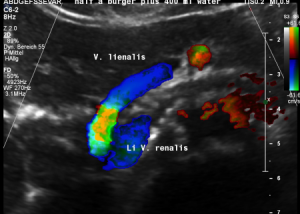
Postprandial compression of the splenic vein by the left renal venous stent
The stent had an unexpected additional drawback: In an upright position it produced a severe compression of the splenic vein. This effect was absent in the supine (lying on the back) position of the patient. This observation corresponded very well with the patient’s description of increasing pain while standing upright.
These images demonstrate a sharp flow acceleration within the splenic vein which is depicted by the ultrasound machine in red and green. The flow velocity is around 200 cm/s which is about 10 times the normal value. This produces a sharp pain within the pressure rise splenic vein as well as in the congested spleen itself. Thus, the patient suffered from circumscribed pain in the upper mid-abdomen as well as in the left hypochondrium, where also the pain from the congested kidney can be felt.
Summary
The patient had undergone a stenting of the left renal vein to reduce the compression of this vessel.
Unfortunately, the stent was too weak to withstand the high pressure of the lordogenetic anterior shift of the aorta. The stent collapsed at 3 locations and became functionally insufficient.
A median arcuate ligament syndrome which contributes substantially to the patient’s symptoms was left undetected.
The dysfunctional stent had an additional detrimental effect. It compressed the splenic vein. This compression was only existence in an upright position of the patient.
Thus, conventional imaging procedures as CT and MRI must miss this functional compression under gravitational stress since the patient is lying on its back in CT and MRI. In just this position no splenic vein compression is visible.
Only functional ultrasound with a thorough search for all vascular compression syndromes can properly grasp all of them. It is the only method to quantify the renal blood flow in different layers of the renal parenchyma to describe the functional state of the stent.
Moreover, functional ultrasound is the only diagnostic imaging method which can be carried out in exactly the circumstances which produce most of the symptoms of the patient.
In this case only in an upright position the severe compression of the splenic vein could be detected.
Example 7: Gravitational stent dysfunction
History
The patient was healthy all her life but started suffering 3 years ago from severe pain in her genitals, mainly on the left side of the perineum and the left side of the rectum with pain after defecation.
The pain in the is genitals permanent but not felt at night. The body posture influences the pain sensations with an amelioration while laying down. There is numbness of the left sole and a tingling sensation in the entire left leg as well as numbness of the left buttock and of the left dorsal upper thigh.
She lost 13 kg during the last year.
In 2018 a pelvic congestion was diagnosed by an MRI and more than 30 coils have been placed into the pelvic veins. Since this not did not result in any improvement, a stent into the left renal vein was placed in May 2019. Unfortunately, the stent had no effect on her suffering.
Physical examination
There is a spontaneous pain in the left perineal region. Here no dilated subcutaneous vessels or a subcutaneous swelling is visible. The palpation of the abdomen does not reveal any pain or lump.
Functional ultrasound
The PixelFlux measurements of the renal parenchyma reveal a severe suppression of the perfusion of the left kidney but only in an upright position:
This reflects very well the symptoms of the patient. She was suffering mainly in an upright or sitting position but felt relief while laying down or sleeping.
The mishappened stent
The stent (3) was too short to stay in the intended location between the aorta (1) and the superior mesenteric artery (2). It slipped towards the right side of the aorta and the left renal vein collapsed again as before the procedure (arrows):
Thus, not surprisingly, the original symptoms reoccurred .
But the dislodged stent was not only ineffective but in addition produced more problems.
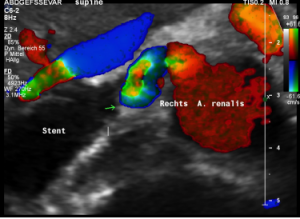
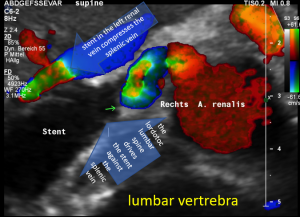
The stent squeezing the right renal artery
The image above shows, how the dislodged left opening of the stent folds the origin of the right renal artery. This buckling artery exerts an additional pressure onto the left renal vein which runs directly in front of the artery.
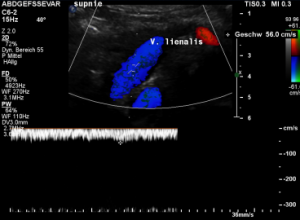
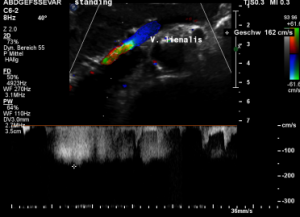
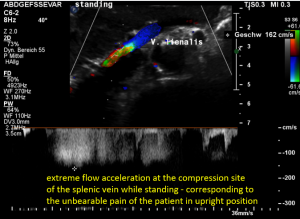
The stent squeezes the splenic vein too-but only while standing upright
While lying down, in the typical position for MRI and CT, no abnormalities of the splenic vein can be detected. The vessel runs untouched by the renal venous stent with a distance to it around 3 mm.
The flow velocity within the splenic vein (vena lienalis) is 56 cm/s.
After assuming an upright position, the liver sank downwards thus pressing the splenic vein against the dislodged stent within the left renal vein:
The flow velocity in the compressed portion of the splenic vein (red and green) increases to 162 cm/s.
Further vascular compression-so far undetected
As usual, additional vascular compressions coexist, as soon as an adult patient presents with one vascular compression.
In this patient a median arcuate ligament syndrome was left undetected but contributed to the symptoms of the patient.
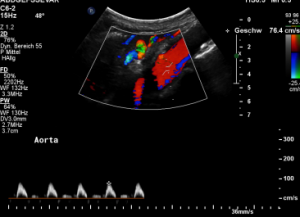
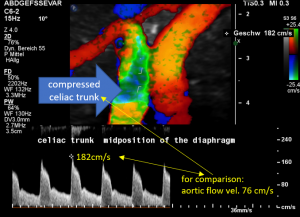
In midposition of the diaphragm the celiac trunk is distorted and compressed thus producing a flow acceleration to 182 cm/s.
Such flow velocities are frequently found in the aorta of young children. In an adult, with increasing age, flow velocities within the aorta drop substantially. In this patient the aortic flow velocity was only 76 cm/s.
Thus, a simple flow measurement within the celiac trunk is not as reliable as a comparison with the blood flow velocity inside the aorta. Only this way a significant flow acceleration as a consequence of the celiac trunk compression can be detected. The pure referral to fixed normal ranges – often 200 cm/s are regarded the upper limit of normality – falls short and leaves the diagnosis undetected.
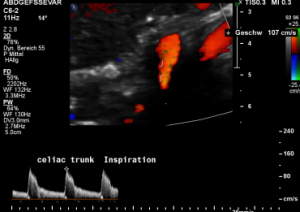
The proof of the causal action of the median arcuate ligament is given by the changing flow velocity in different diaphragmatic positions. Here the flow velocity drops in inspiration to 107 cm/s and the turbulences (green) disappear since the pressure onto the vessel is reduced.
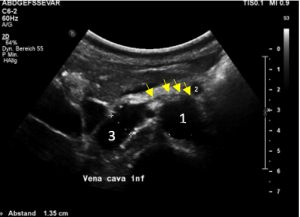
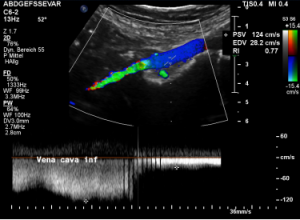
Compression of the vena cava inferior by bowel loops
The strong lumbar lordosis lifts up the Vena cava and presses it against bowel loops. The patient felt this as severe pain in her right mid abdomen when she felt her bowel loops filling.
Here a sharp flow acceleration is depicted in green corresponding to an increased flow velocity to 124 cm/s. Within the white part of the vena cava (plain blue) the flow velocity is substantially less around 30 cm/s. Exactly at the location of the compression of the vena cava the patient reported severe post-prandial pain.
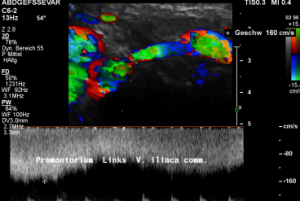
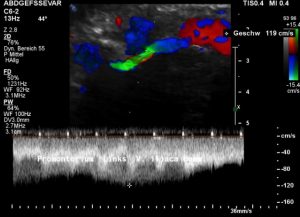
A May-Thurner syndrome was left undetected so far
The left common iliac vein is an important vessel to drain the left pelvic hemisphere.
It is even more important than the left sided pelvic veins have to take up blood from collateral pathways of the compressed left renal vein.
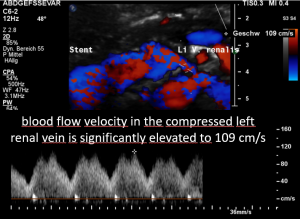
Then a compression of this vessel by the uplifted sacral bone (1) at the crossing with the overriding right iliac artery (2) produces not only pain within the left pelvis but increases the pain in the left renal fossa as well as along the course of the dilated left renal vein.
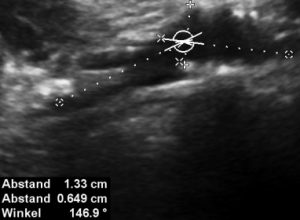
Here, the compression of the left iliac vein (3) because of its uplifting by the prominent sacral bone (1) and the crossing right common iliac artery (2) produces a flow acceleration from 22 (not shown) to 160 cm/s.
Summary
If a compression of the left renal vein is found, other compression syndromes should be expected.
The patient suffered from 3 vascular compressions in the beginning:
- May-Thurner constellation
- Celiac artery compression (M ALS)
- Left renal vein compression (also known as nutcracker syndrome)
Only one compression had been detected and was treated with a stent – the left renal vein compression. The pelvic veins, which had responded to the additional volume from the collateral pathways of the left renal vein had been embolized. So, the pressure in the left renal vein became unbearable, as soon as the stent slipped out of the compression site.
Unfortunately, the stent not only became insufficient but caused two other vascular compressions, the folding of the origin of the right renal artery, which so far is not functional relevant for the blood flow towards the right kidney, and the compression of the splenic vein while standing upright.
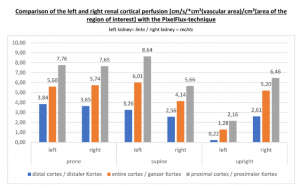
The upright position further increases the perfusion problems of the left kidney. This can be shown only with the PixelFlux technique.
Such an information is valuable because it evokes further pathophysiological considerations.
In this case it seems possible that some of the congested left renal blood is bypassed via collaterals towards the spleen. So, the function of the splenic vein may become important for the drainage of the left kidney.
Alas, in an upright position the stent produced a compression of the splenic vein thus obstructing a possible bypass for the persistently compressed left renal vein.
The sharp drop of left renal perfusion in an upright position occurs since other relevant collateral pathways have been embolized.
Example 8
Left renal venous stent penetrates the wall of the inferior vena cava and compresses the splenic vein – while standing
History
The patient, now 77 years old, was healthy all her life but started suffering 3 years ago from severe pain in her genitals, mainly on the left side of the perineum and the left side of the rectum with pain after defecation.
From then on, she also developed bouts of high blood pressure at maximum more than 200 mmHg systolic pressure.
The pain in the genitals permanent but not felt at night. The body posture influences the pain sensations with an amelioration while laying down. There is numbness of the left sole and a tingling sensation in the
entire left leg as well as a numb left buttock and of the left dorsal upper thigh. A year ago, a pelvic congestion was diagnosed by MRI and more than 30 coils have been placed into the pelvic veins. Since this not did not result in any improvement, a stent was placed in May of this year into the left renal vein. Unfortunately, the stent did move and had no effect on her suffering.
Clinical examination
There is a spontaneous pain in the left perineal region. Here no dilated subcutaneous vessels or a subcutaneous swelling is visible. The palpation and auscultation of the abdomen is unremarkable.
Sonographic findings
A May-Thurner constellation is demonstrated by compression of the left common iliac vein and flow acceleration underneath the crossing of the right common iliac artery
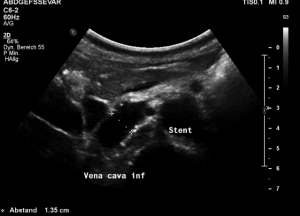
The stent was squeezed out of the aorto-mesenteric angle by the lordotic spine and punched into the inferior vena cava.
The compression of the left renal vein persists (yellow green signal)
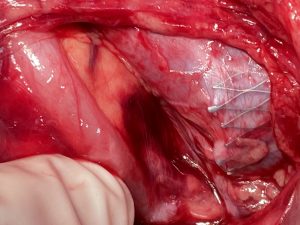
Intraoperatively the sharp triangular mesh of the stent can be seen penetrating the wall of the vena cava like a hollow chisel (yellow arrows)
Additionally, a celiac artery compression was found (overlooked so far – but no incidental finding).
Moreover, the stent also compresses the splenic vein.
Its devastating effect fully develops while standing upright.
Functional effect of postural changes
Only the PixelFlux measurements can demonstrate the true effect of vascular compressions. The driving force of all venous pain is volume overload. The left kidney is abundantly perfused. Consequently, the compression of the left renal vein forces huge amounts of blood to travel through tiny veins in collateral circulations. Such volume overload produces severe pain. The PixelFlux measurement in this case clearly demonstrate a devastating effect of an upright posture. The patient could not tolerate to stand but had to lay down for pain relief.
The PixelFlux measurements show a significant breakdown of left renal parenchymal perfusion in an upright body posture compared to supine and prone horizontal body positions.
Only by PixelFlux measurements the causal connection of the venous compression and the pain of the patient can be proven.
Intraoperative images
Intraoperative images from the patient with a stent in the left common iliac vein to treat a May-Thurner constellation:
Medical history
The patient had been operated in July 2019 to correct a median arcuate ligament syndrome but unfortunately felt no relief after this operation. In October 2019, a nutcracker syndrome was treated by a left renal transplantation towards the left renal fossa. This provided some relief of her pre-existing left flank pain for several months. But then the left flank pain reoccurred. In May 2020 a duodeno- jejunostomy was performed to treat a Superior mesenteric artery syndrome which was diagnosed on grounds of a narrow aorto-mesenteric angle (3 mm passage for the duodenum/19°). Interestingly, preoperatively the contrast medium did not provoke a dilatation of the descending part of the duodenum. In July this year a May-Thurner constellation was treated by insertion of a stent into the left common iliac vein. After this stent insertion still a dull pain in the left iliac fossa and in the lower abdomen persists. Moreover, the preoperative symptoms, consisting of nausea, epigastric pain, weight loss and vegetative symptoms with heart rates around 120/min in an upright position, sudden flushing and sweating are described again. The epigastric pain is a constant one at the level of 7/10 but increases post-prandially to 8/10. The patient reports an early satiety and inability to ingest sufficient amount of food by mouth. But now even fluids are not well tolerated, and the intake of any food is responded by belching, bloating and vomiting. So, the patient has to fight a constant tendency to lose weight and is fed via an intravenous line for ¾ of her daily nutrition. The suspicion of a compression of one ending of her pelvic stent was raised. Moreover, a haematuria persists after transplantation of the left kidney as well as abdominal tension and severe pain in the abdomen in general but most pronounced in the left lower quadrant. At the apex of her pain she must expect to faint. That is why she stays in bed most of the day and cannot attend school for 3 years now. She suffers from severe nuchal headaches which also radiate towards the front, this requires analgesic treatment 3 times a day since the pain reaches 8/10. A frequent diarrhoea with interspersed phases of constipation is observed as well. The patient lost weight from 127-106 pounds (57.8 kg to 48.08 kg) and has an actual weight of 121 (54.8 kg) with a height of 5 feet 7 inches (170 cm). The today’s examination aims at a reevaluation of the operation sites and finding a reason for her a persisting severe pain and general suffering.
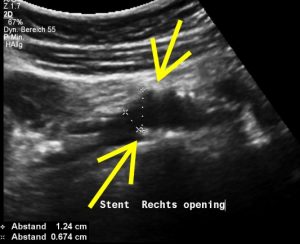
Left-sided opening of a stent within the left common iliac vein – the metal tips of the stent penetrate the wall of the iliac vein and poke through the wall of the vena cava inferior
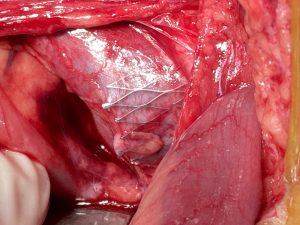
The penetrating stent caused severe pain and was insufficiently perfused – this was only demonstrated by a colour Doppler sonographic volume flow measurement and was invisible with CT
Here are the corresponding preoperative ultrasound images. They clearly predict the intraoperative findings.
The stenting does not align with the curving course of the uplifted left common iliac vein-this is the very reason of the penetration and pain in the patient
Synopsis
The pain in the genitals is produced by the pelvic congestion as a consequence of the May-Thurner constellation. The extreme pain in the upper abdomen is a result of the manifold failure of the stenting, resulting in:
- persisting compression of the left renal vein
- Laceration of the wall of the vena cava
- Compression of the splenic vein
In addition, the so far overlooked celiac artery compression contributes to vegetative symptoms and pain in the epigastric angle.
Learning points
- The placement of a stent in front of a constantly moving bony structure (lumbar spine or sacral bone, constantly moving during daily activities) which compresses the stent is no good idea.
- The stent is forced to break or to move, both resulting in pain and disturbed blood flow.
- One compression syndrome rarely runs alone. A successful treatment strategy has to consider the entire spectrum of vascular compressions
References
Many papers describe short term results of endovascular stenting in venous compression syndromes of the abdomen. The technical success rate is invariably high. Long term results are not as frequent reported, especially in non-thrombotic patients.
Here is a selection of papers fulfilling this criterion:
Predictors of Disease Recurrence after Venoplasty and Stent Placement for May-Thurner Syndrome J Vasc Interv Radiol. 2019 Oct;30(10):1549-1554.
- Fifty-nine consecutive patients (age, 47 y ± 15; 93% female) were identified who had undergone endovascular stent placement for MTS
- Disease recurrence, defined as symptom recurrence following initial postprocedural resolution, was observed in 38% of patients.
Impact of degree of stenosis in May-Thurner syndrome on iliac vein stenting J Vasc Surg Venous Lymphat Disord. 2019 Mar;7(2):195-202
- Normal luminal diameters and areas were defined as 12 mm and 125 mm2, 14 mm and 150 mm2, and 16 mm and 200 mm2in the common femoral, external iliac, and common iliac veins, respectively. Mild (<60%), moderate (60%-89%), and severe (>90%) compression groups were defined
- Long term, patients with ≥90% initial MTS stenosis experience recurrence of symptoms.
Endovascular Stent Placement for May-Thurner Syndrome in the Absence of Acute Deep Vein Thrombosis.J Vasc Interv Radiol. 2016 Feb;27(2):167-73.
- All patients (N = 45) undergoing stent placement for May-Thurner syndrome (MTS) without an associated acute thrombotic event between 2007 and 2014 were retrospectively reviewed
- 68% of patients (23 of 34) had clinical success with relief of presenting symptoms
Contralateral Deep Vein Thrombosis after Iliac Vein Stent Placement in Patients with May-Thurner Syndrome. J Vasc Interv Radiol. 2018 Jun;29(6):774-780
- Data of 111 patients (women: 73%) who had CIV stent implantation for symptomatic MTS at a single center were retrospectively analyzed.
- Ten patients (9%, men/women: 4/6) exhibited contralateral DVT at a median timing of 40 months (range, 6-98 months).
- Contralateral DVT after CIV stent implantation has a relatively high incidence and often occurs late during follow-up.
J Vasc Surg. 2005 Aug;42(2):275-80.
- From November 2002 to September 2004, five women (mean age, 34.7 years) were admitted for endovascular treatment of a nutcracker syndrome
- Technical success was achieved in all cases. One case of stent migration occurred:
- Pelvic pain recurred in one patient who had initially improved, and endometriosis was diagnosed 15 months after the procedure.
- Two other patients, who received 40-mm-long stents, had a secondary recurrence of the symptoms caused by stent dislodgement.
- The two other patients were asymptomatic.